Abbreviations
PBC, primary biliary cholangitis; PPAR α/δ, peroxisome proliferator-activated receptor alpha + delta
Reference
- IQIRVO® (elafibranor) Summary of product characteristics (SmPC). 2024.
This section of the website is for UK Healthcare Professionals only. If you are not a Healthcare Professional, please click here.

Mechanism of action
While the exact mechanism by which IQIRVO exerts its therapeutic effects in patients with PBC is not fully understood, it is thought that PPAR-α and PPAR-δ are key regulators of bile acid homeostasis, inflammation and fibrosis.

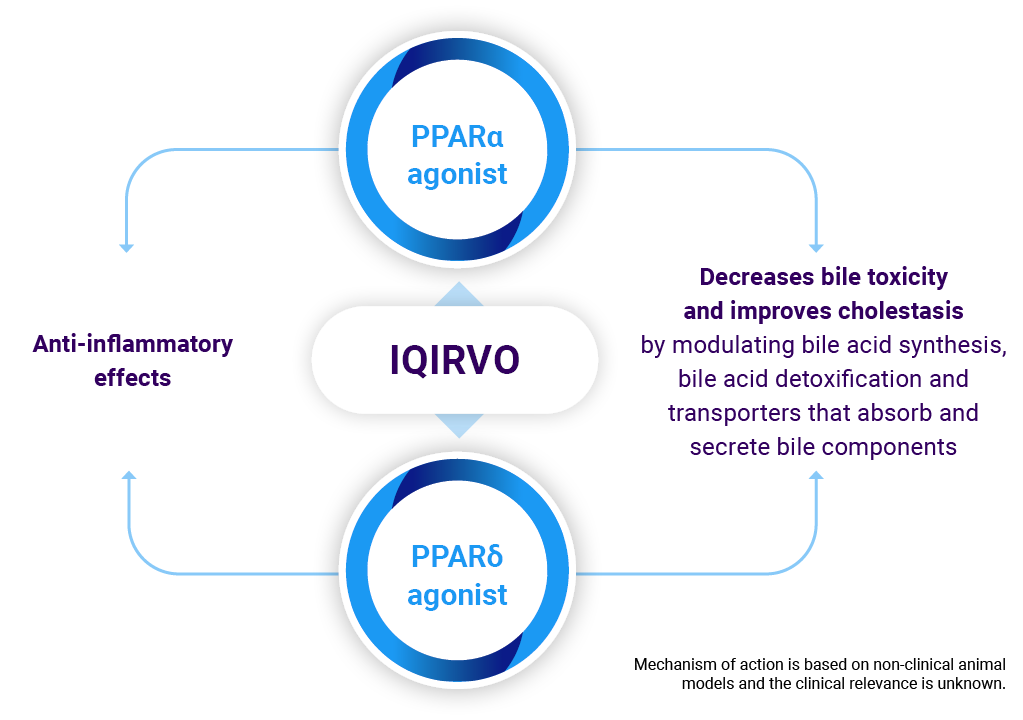
PBC, primary biliary cholangitis; PPAR α/δ, peroxisome proliferator-activated receptor alpha + delta