
IQIRVO
Safety data
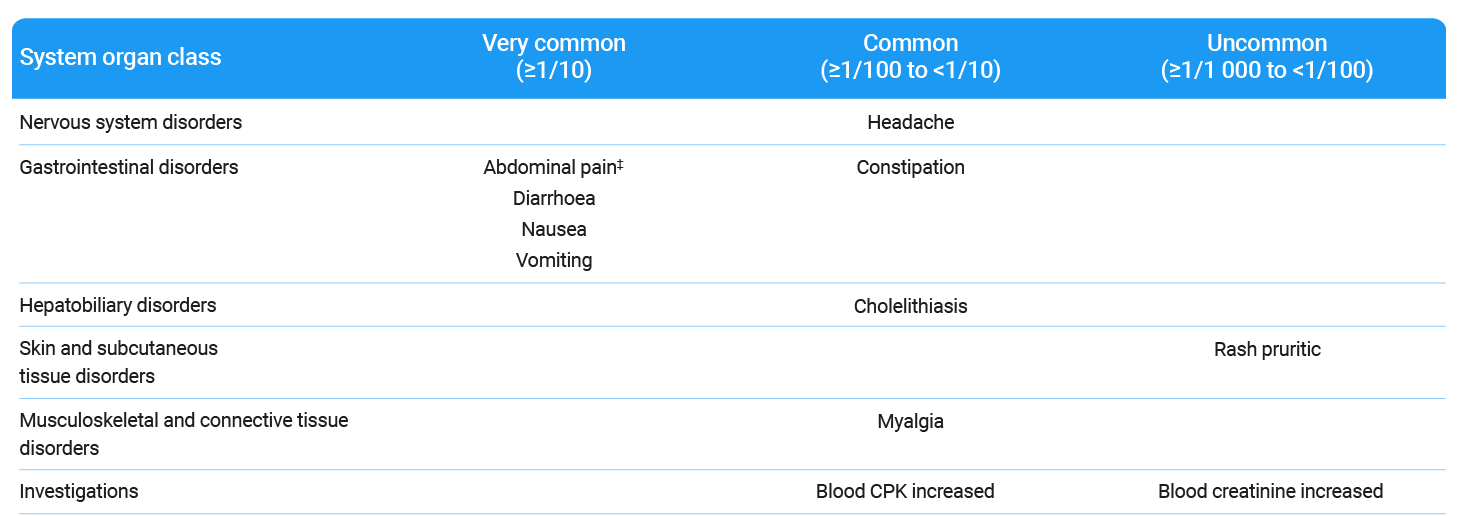
Within the system organ class, the adverse reactions are listed by frequency using the following categories: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1 000 to <1/100), rare (≥1/10 000 to <1/1 000), very rare (<1/10 000), not known (cannot be estimated from the available data).2
Special considerations and precautions for IQIRVO + UDCA use include:2
Liver-related events
- Clinical and laboratory assessment of liver function should be done prior to initiation of IQIRVO treatment and thereafter according to routine patient management.
- If increases in liver biochemical tests and/or liver dysfunction are observed, prompt investigation of the cause is recommended and interruption of IQIRVO treatment should be considered.
Fertility
- There is limited data on the effect of IQIRVO on fertility are available. Animal studies do not indicate any direct or indirect effects on fertility or the ability to reproduce.
Pregnancy: Embryo-Foetal Toxicity
- There is limited amount of data from the use of IQIRVO in pregnant women. Studies in pregnant animals have shown reproductive toxicity. IQIRVO is not recommended during pregnancy.
Women of childbearing age/contraception
- The pregnancy status of patients of childbearing potential should be checked prior to initiation of IQIRVO treatment.
- The use of IQIRVO is not recommended in women of childbearing potential not using effective contraception.
- Women of childbearing potential should continue to use effective contraception during and up to at least for 3 weeks following the final dose of IQIRVO.
Lactation
- It is unknown whether IQIRVO or its metabolites are excreted in human milk. A risk to the suckling child cannot be excluded.
- IQIRVO should not be used during breastfeeding and for at least 3 weeks following the last dose.
Hypersensitivity reactions
- If severe hypersensitivity reactions occur, permanently discontinue IQIRVO.
- If a mild or moderate hypersensitivity reaction occurs, stop IQIRVO and treat promptly then continue with the follow up until resolution of the signs and symptoms.
Elevated blood CPK and muscle injury
- CPK should be evaluated prior to initiation of IQIRVO treatment to determine the baseline CPK level and thereafter, according to the routine patient management.
- Caution should be used in patients with predisposing factors including old age (>65 years), hypothyroidism, personal or familial history of hereditary muscular disorders, severe infection, trauma, surgery, disturbances of hormone or electrolyte imbalance or alcohol abuse.
- Periodic CPK measurements may be considered in patients starting IQIRVO treatment, especially those on concomitant HMG-CoA reductase inhibitors.
- Patients on IQIRVO treatment should be advised to report promptly any unexplained muscle symptoms such as pain, soreness, or weakness, especially if accompanied by malaise or fever.
- If increases in CPK or unexplained signs and symptoms of muscle injury are observed, prompt investigation of the cause is recommended. Interruption of IQIRVO treatment should be considered and levels monitored as per standard medical practice.
Fractures
- Consider the risk of fracture in the care of patients treated with IQIRVO and monitor bone health according to current standards of care.
Elderly (over 80 years)
- There is no supporting data on using IQIRVO in those over 80 years of age.
Interaction with fibrates
- Concomitant administration is not recommended.
Interaction with other medicinal products and other forms of interaction
- Based on in vitro and in vivo studies, no clinically relevant drug-drug interaction is expected by co-administering IQIRVO with any other medicinal products.
For full list of special warnings and precautions for use, please refer to the SMPC before prescribing IQIRVO.
Footnotes
*Includes abdominal pain upper and abdominal pain lower.2
Abbreviations
AE, adverse event; CPK, creatine phosphokinase; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A;
UDCA, ursodeoxycholic acid.
References
- Kowdley KV et al. N Engl J Med. 2024;390(9):795–805.
- IQIRVO® (elafibranor) Summary of product characteristics (SmPC). 2024.
Resources
- PBC
- IQIRVO MoA
- The ELATIVE trial: Study design & baseline characteristics
- The ELATIVE trial: Efficacy
- The ELATIVE trial: Safety data
- IQIRVO Safety Data
- Dosing
- Resources
