
IQIRVO ELATIVE trial
Study design and baseline characteristics
The ELATIVE trial: study design
The ELATIVE trial is a double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of IQIRVO in patients with PBC and inadequate response or intolerance to UDCA1
- IQIRVO was tested as an add-on to UDCA: 95% (153/161) of patients were receiving concurrent UDCA therapy1

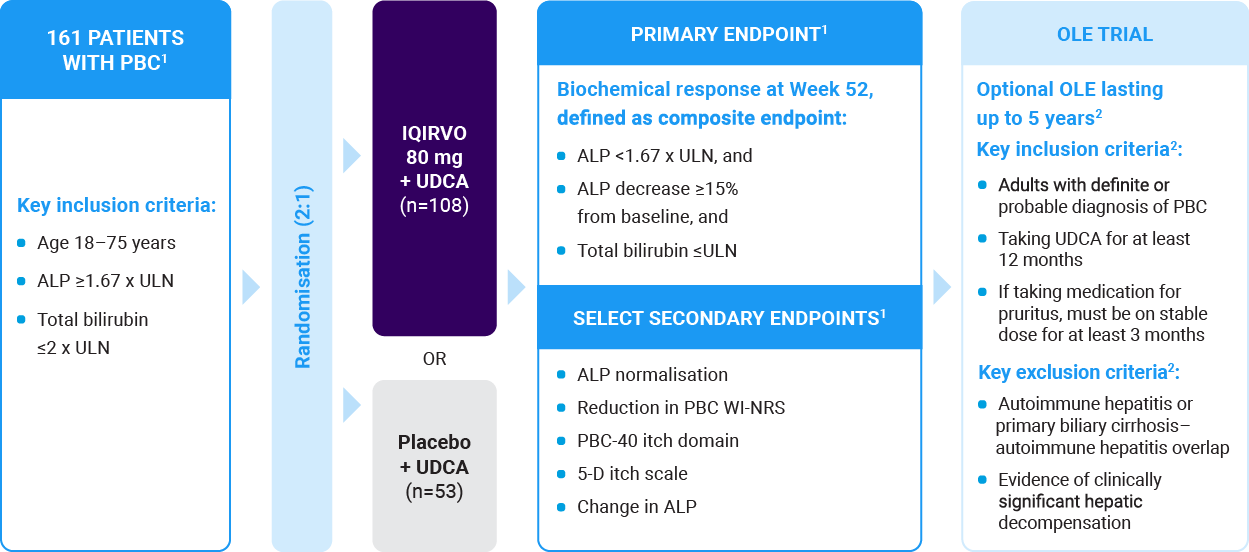
Key secondary endpoints (normalisation of ALP at Week 52, and the change from baseline in the WI-NRS score through Week 52 and through Week 24) were assessed with the use of a pre-specified fixed sequence testing approach, at a two-sided alpha of 0.05, until a non-significant result was encountered.
Other secondary end points are reported as point estimates and 95% confidence intervals, which were not adjusted for multiple testing.
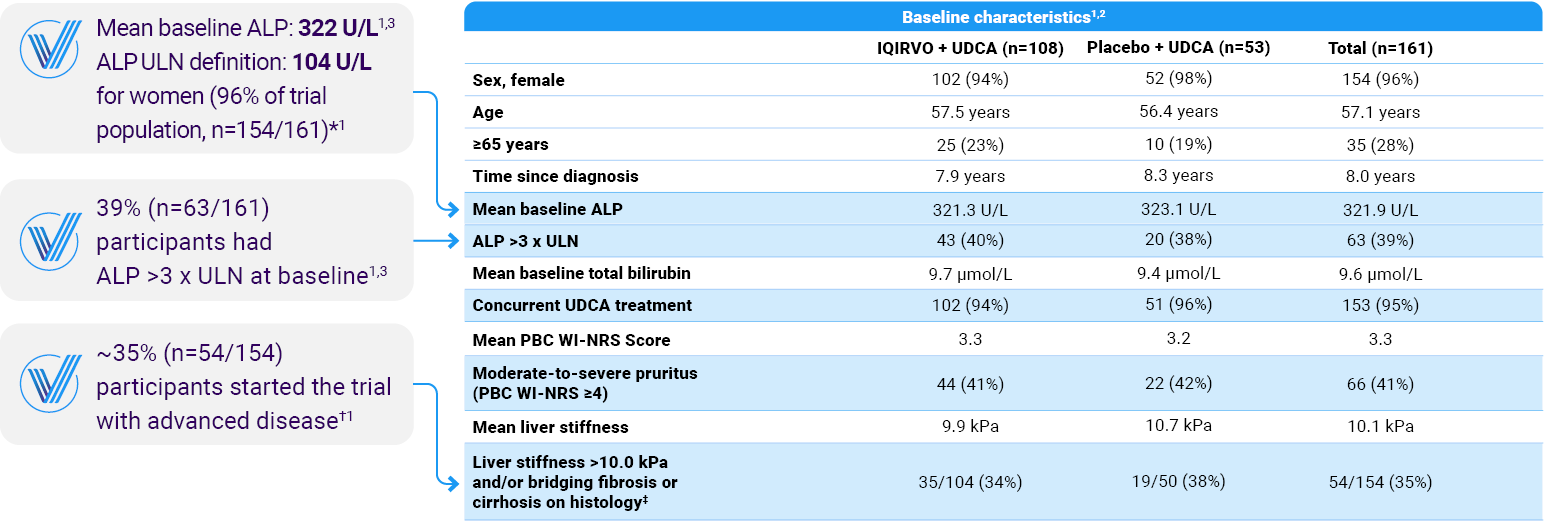
Footnotes
*ALP ULN was defined as 104 U/L for women (154/161 participants) and 129 U/L for men (7/161 participants).1
†Advanced disease stage defined as liver stiffness at baseline >10 kPa and/or bridging fibrosis or cirrhosis on histology.2
‡The presence or absence of bridging fibrosis or cirrhosis was determined by histologic findings in the patients who underwent a liver biopsy.1
Abbreviations
ALP, alkaline phosphatase; kPa, kilopascals; mg, milligram; OLE, open-label extension; PBC, primary biliary cholangitis; PBC WI-NRS, primary biliary cholangitis worst itch numeric rating scale; UDCA, ursodeoxycholic acid; ULN, upper limit of normal; U/L, units per litre.
References
- Kowdley KV et al. N Engl J Med. 2024;390(9):795–805.
- Kowdley KV et al. Supplement to: N Engl J Med. 2024;390(9):795–805.
- IQIRVO® (elafibranor) Summary of product characteristics (SmPC). 2024.
Resources
- PBC
- IQIRVO MoA
- The ELATIVE trial: Study design & baseline characteristics
- The ELATIVE trial: Efficacy
- The ELATIVE trial: Safety data
- IQIRVO Safety Data
- Dosing
- Resources
